Is Eli Lilly Stock a Buy, Sell, or Fairly Valued After Its 2023 Rally?
With a 30% rally on optimism about two key drug lines, here’s what we think of Eli Lilly stock.

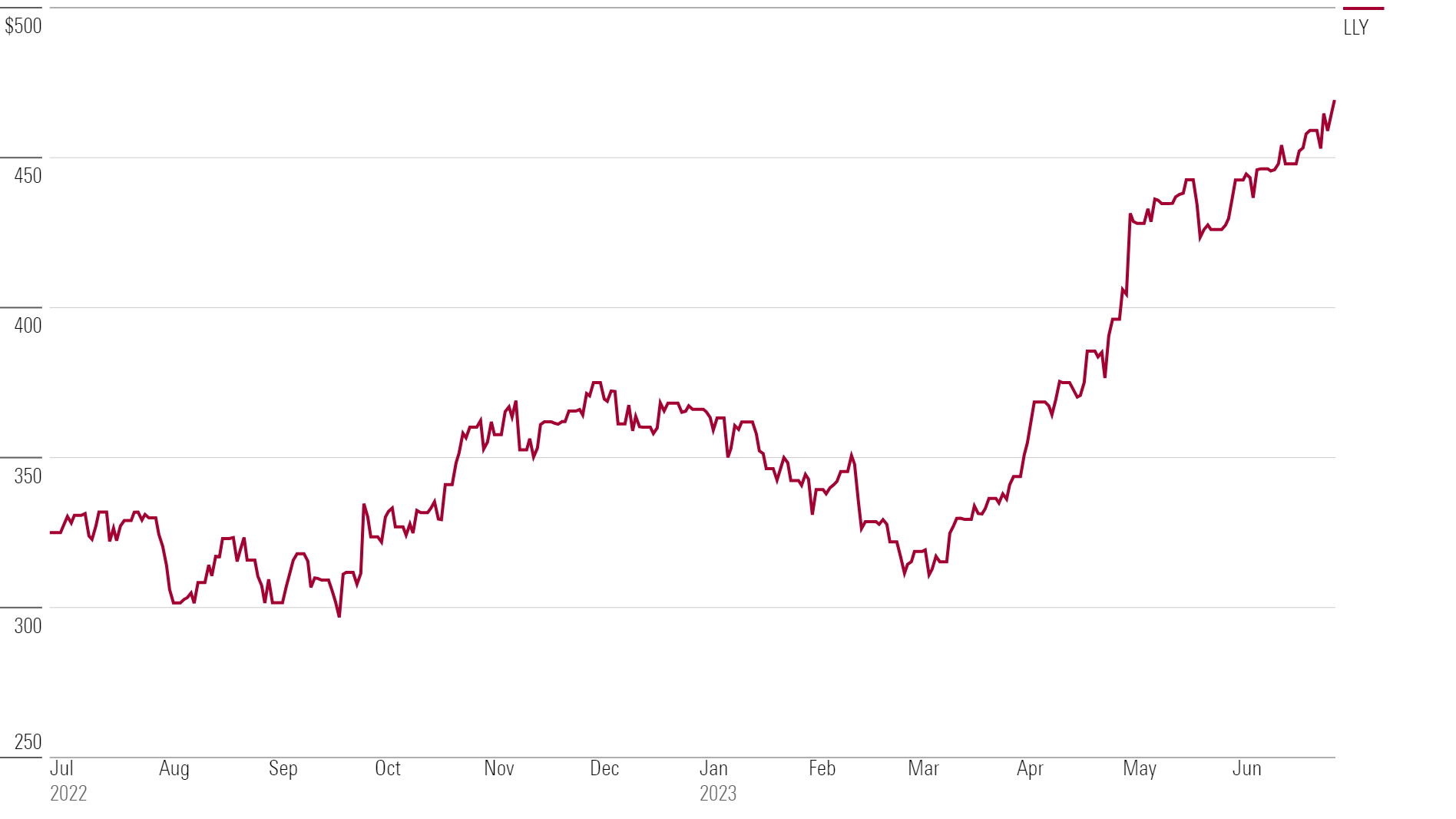
Eli Lilly’s LLY stock has rallied 30% this year, in part on news about two potential big new product lines. Here’s what we think of Eli Lilly stock.
Eli Lilly Stock at a Glance
- Fair Value Estimate: $368.00
- Morningstar Rating: 2 stars
- Morningstar Economic Moat Rating: Wide
- Morningstar Uncertainty Rating: High
Eli Lilly Stock Update
The key driver of the rally in Eli Lilly stock has been optimism about the potential for two of the company’s drugs: Mounjaro (tirzepatide) for weight loss and donanemab for early Alzheimer’s. We are more bullish than the consensus about both of these drugs, as we believe the market is getting too bullish—more than what is implied by consensus expectations.
Here are our thoughts on the prospects for these drugs:
Mounjaro: We have been increasing our projections for this drug based on its very strong recent news flow, especially in the weight loss area. Mounjaro is a once-weekly dual incretin drug that stimulates hormones that control blood sugar levels and reduce appetite. Phase 3 data in diabetes from late 2020 and 2021 has been excellent, leading to approval for its use with diabetes in 2022. We expect this data to allow Mounjaro to remain competitive with Novo Nordisk’s semaglutide. Additional phase 3 data in heart failure (expected in 2024), sleep apnea (2024), and a cardiovascular outcomes study (2024) should likely add to the strong data already reported.
Donanemab: This drug targets a protein called amyloid, which is thought to be a cause of Alzheimer’s disease. Overall, the recent dataflow has been fine, with the minor setback of the company not being able to get approval on early data, but it’s shown good phase 3 efficacy (albeit with some side effect issues).
While donanemab’s phase 2 study was not a major success, the drug slowed clinical decline in patients by 32% (using the iADRS metric), but the benefit was barely statistically significant, with a p-value of 0.04. The drug also failed to show a statistically significant benefit on the CDR-SB metric, which we see as a more currently accepted measurement. In the phase 3 study, the drug impressively met all primary and secondary endpoints related to slowing cognitive and functional decline. However, the side effect of serious ARIA (swelling of the brain) occurred in 1.6% of patients and led to at least two deaths. Despite this, we expect the drug will gain approval in early 2024 based on its strong efficacy.
On market sizing, we expect strong donanemab traction with Alzheimer’s patients who have intermediate tau accumulation (30%-45% of the market), as this biomarker level looks most poised to signal a response to the drug. However, utilization likely faces diagnosis challenges, given the lower rate of identifying Alzheimer’s patients (especially by tau levels). We expect strong pricing similar to Aduhelm and a net price of $30,000 in the United States, with a market share of close to 10% of diagnosed patients supporting an $8 billion global peak sales estimate for the drug by 2030. Meanwhile, donanemab looks well positioned to largely split the Alzheimer’s market with Biogen’s competitive drug Leqembi.
Eli Lilly has an excellent management team. However, even with this and the company’s great portfolio of drugs with limited patent losses, we still don’t find the valuation as compelling as the consensus does.
Fair Value Estimate for Eli Lilly Stock
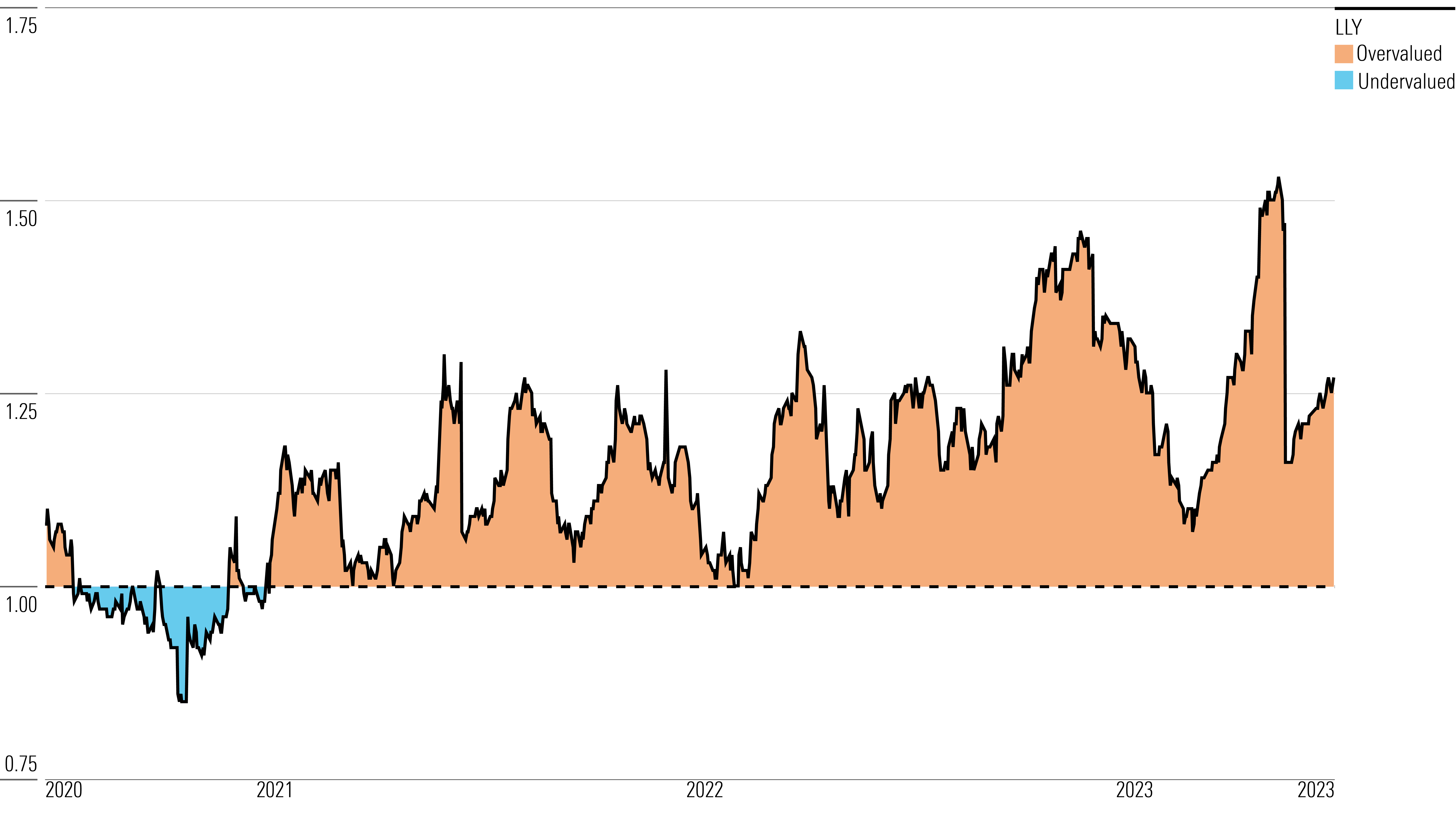
With its 2-star rating, we believe Eli Lilly’s stock is overvalued compared with our fair value estimate.
We have increased our fair value estimate to $368 from $289 per share, largely based on increased projections for Mounjaro and the diabetes drug orforglipron. While Mounjaro sales have yet to fully materialize (partly due to insurance still expanding coverage), we expect this drug to become Lilly’s largest product over the next five years, based on a leading efficacy profile in diabetes and weight loss. We believe Mounjaro has a peak annual sales potential above $25 billion. The drug entered the market in 2022 for diabetes treatment, and we expect a label expansion into obesity treatment in 2023.
Additionally, we expect orforglipron to develop into a major drug based on the convenience of its oral administration. Its efficacy looks positioned to match Novo Nordisk’s competitive drugs Wegovy and potentially high-dose oral semaglutide, and as a non-peptide agonist, the drug doesn’t require any special delivery technology.
Read more about our fair value estimate for Eli Lilly stock.
Eli Lilly Historical Price/Fair Value Ratios
Economic Moat Rating
Patents, economies of scale, and a powerful distribution network support Eli Lilly’s wide moat.
Lilly’s patent-protected drugs carry strong pricing power, which enables the firm to generate returns on invested capital in excess of its cost of capital. Further, the patents give the company time to develop the next generation of drugs before generic competition arises. Lilly’s diversified product portfolio means the company’s top drugs represent only a moderate amount of total sales. The largest drug, Trulicity, represents almost 20% of total sales, which sets up manageable cash flow declines as new products mitigate the generic competition. Also, Lilly’s operating structure allows for cost-cutting after patent losses to reduce the margin pressure from lost high-margin drug sales.
Overall, Lilly’s established product line creates the enormous cash flows needed to fund the average $800 million development cost of each new drug. In addition, the company’s powerful distribution network sets it up as a strong partner for smaller drug companies that lack its resources. Lilly’s entrenched insulin franchise creates an added layer of competitive advantage, as interchangeable insulin competition seems many years away due to the complexity of gaining generic approval for insulin and the high cost to build the needed economy of scale for insulin production.
Read more about Eli Lilly’s moat rating.
Eli Lilly Stock Price
Risk and Uncertainty
We have increased Eli Lilly’s Uncertainty Rating to High from Medium based on an increasingly variable outcome for several key drug launches. Mounjaro is likely to develop into a major new drug, but its cone of uncertainty is higher since several variables affect the sales potential for the weight loss indication, including level of insurance coverage and pricing. Donanemab holds tremendous potential, but its outlook also has a wide range of outcomes, since the market potential could be very large but the visibility on market uptake is less clear.
With these two drugs representing close to half of Lilly’s projected sales by the end of the next 10 years, we believe a High Uncertainty Rating is appropriate. Most big biopharma firms tend to have Medium Uncertainty Ratings. Beyond product-specific uncertainties, Lilly faces tough competition from generics manufacturers and brand-name drugmakers. The company encounters considerable regulatory and legal risks, including product approvals, patent challenges, and liability lawsuits.
LLY Bulls Say
- Lilly is developing donanemab, a new Alzheimer’s drug that could become a major blockbuster, especially since the FDA appears to have a lower threshold for approval for this disease.
- Lilly’s cancer drug Verzenio reported strong data in early-stage breast cancer, opening up the potential to be the first CDK4/6 drug to launch in this multi-billion-dollar market.
- Lilly is creating the next generation of diabetes and weight loss drugs that hold major market potential, given the high prevalence of these diseases.
LLY Bears Say
- The risks to success for donanemab remain high, both in clinical development and insurance coverage.
- Several of Lilly’s next-generation diabetes drugs could lead to the cannibalization of its current approved drugs.
- Competition for weight-loss drug Mounjaro could significantly increase over the next three years.
This article was compiled by Tom Lauricella.
Get access to full Morningstar stock analyst reports, along with data and tools to manage your portfolio through Morningstar Investor. Learn more and start a seven-day free trial today.
MORN DODFX VINIX VWILX TSVA EGO WU Brightstart429plan MRO VZ MOAT T NKE CMCSA GOOG
The author or authors do not own shares in any securities mentioned in this article. Find out about Morningstar’s editorial policies.

/s3.amazonaws.com/arc-authors/morningstar/a90c659a-a3c5-4ebe-9278-1eabaddc376f.jpg)
/cloudfront-us-east-1.images.arcpublishing.com/morningstar/6ZMXY4RCRNEADPDWYQVTTWALWM.jpg)
/d10o6nnig0wrdw.cloudfront.net/05-20-2024/t_dc0763464c0a4191b876af1624b96f43_name_file_960x540_1600_v4_.jpg)
/cloudfront-us-east-1.images.arcpublishing.com/morningstar/IPKX4IWSDBD3VJC2Y34W6STFL4.jpg)
:quality(80)/s3.amazonaws.com/arc-authors/morningstar/a90c659a-a3c5-4ebe-9278-1eabaddc376f.jpg)