Clarity's theranostic prostate cancer trial advances to multi-dose phase
Clarity's theranostic prostate cancer trial advances to multi-dose phase
PR Newswire
SYDNEY, March 15, 2024
Highlights
- Cohort 3 of the theranostic SECuRE trial investigating 64Cu/67Cu-SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has been completed in 6 participants who received therapy with 67Cu-SAR-bisPSMA at the highest single dose level of 12GBq.
- No dose limiting toxicities (DLTs) have been reported in cohort 3 to date.
- An overall safety review of all cohorts 1, 2 and 3 (4, 8 and 12GBq single dose, respectively) showed a favorable safety profile, with most adverse events (AEs) being mild or moderate.
- The Safety Review Committee (SRC) has recommended that the trial progresses to cohort 4, a multi-dose phase, at the highest dose investigated in the study (12GBq).
- Although the last participants in cohort 3 only completed dosing in January 2024, 60% of participants across all cohorts so far showed reductions in prostate-specific antigen (PSA) levels of greater than 35% from a single dose of 67Cu-SAR-bisPSMA. Twenty-seven percent of participants showed reductions in PSA levels of greater than 80%.
- Among the participants from cohorts 2 and 3, almost 80% showed reductions in PSA levels of greater than 35% from a single dose of 67Cu-SAR-bisPSMA, and 44% showed reductions in PSA levels of greater than 80%.
- Participants treated in the trial to date have received multiple lines of therapy prior to their recruitment into the study, including androgen deprivation therapy (ADT), androgen receptor pathway inhibition (ARPI) therapy, investigational agents, chemotherapy and other radioligand therapies such as alpha and beta-emitters (225Ac and 177Lu-based therapies, respectively).
- Cohort 3 participants had the highest number of pre-treatments prior to entering the study across all cohorts, with most patients in this cohort receiving 5 or more lines of therapy. The number of lines of prior therapy in cohort 3 was almost double compared to cohort 2. Cohort 3 also had the highest pre-treatment median PSA across all cohorts (140.3 ng/ml, with a patient having the highest level in the cohort at 781.8 ng/ml).
- Recruitment has opened for cohort 4, the first multi-dose cohort in the SECuRE trial, at clinical sites in the US. Participants in cohort 4 will receive multiple treatment cycles at the dose level of 12GBq of 67Cu-SAR-bisPSMA. All available patient slots for the first part of cohort 4 have been allocated and screening activities have commenced.
SYDNEY, March 15, 2024 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce the successful completion of cohort 3 and advancement to cohort 4, the first multi-dose cohort in the SECuRE trial.
The SECuRE trial (NCT04868604)[1] is a Phase I/IIa theranostic trial for identification and treatment of Prostate-Specific Membrane Antigen (PSMA) expressing mCRPC using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation trial with a cohort expansion involving up to 44 patients in the US. The aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
Cohort 3 of the dose escalation phase of the trial, where 6 participants received a single administration of 12GBq of 67Cu-SAR-bisPSMA, has been successfully completed. No DLTs have been reported in any of the participants dosed in this cohort to date. The SRC, responsible for assessing safety of participants and overseeing the general progress of the trial, has assessed the data and recommended progressing the trial to cohort 4 where participants will be treated with multiple therapy cycles of 67Cu-SAR-bisPSMA at a dose level of 12GBq, the highest dose level in the dose escalation phase of the trial.
Anti-tumour effect of 67Cu-SAR-bisPSMA
All participants in the SECuRE trial had advanced prostate cancer (stage IV, mCRPC). Assessment of the baseline characteristics of these patients showed that they were heavily pre-treated before entering the study, having received multiple therapies for their disease. Those treatments included ADT, ARPI, several investigational agents (targeting different pathways of the cancer), chemotherapy and other radioligand therapies. Most trial participants had received chemotherapy (67%, 10/15) and the median number of lines of therapy prior to receiving 67Cu-SAR-bisPSMA was 4. The median PSA at study entry was 117.1 ng/ml (range 0.11-1,494.2).
Preliminary data shows that despite having high levels of PSA and having received multiple treatments, 60% (9/15) of participants across all cohorts (including the lowest dose cohort of 67Cu-SAR-bisPSMA at 4GBq) showed reductions in PSA levels of greater than 35% from a single therapy cycle of 67Cu-SAR-bisPSMA. PSA reductions of greater than 80% were seen in 27% of all trial participants. In cohorts 2 and 3 (8 and 12GBq, respectively), PSA reductions of greater than 35% were observed in almost 80% (78%, 7/9) of participants and PSA was reduced by greater than 80% in 44% (4/9) of participants so far.
Participants in cohort 3 had the highest median baseline PSA and the highest median number of systemic therapies across all cohorts (median baseline PSA 122.6, 47.2 and 140.3 ng/ml; median lines of therapy 4, 3 and 5.5; cohorts 1, 2 and 3, respectively). Nevertheless, two-thirds (67%) of participants in this cohort so far have shown reductions in PSA greater than 35%, with the last participants in this cohort dosed in January 2024. Importantly, a single dose of 12GBq of 67Cu-SAR-bisPSMA was effective in reducing PSA levels in the majority of these patients despite receiving the most lines of prior therapy (Figures 1 and 2).
67Cu-SAR-bisPSMA has a favorable safety profile
The overall analysis of all cohorts showed that 67Cu-SAR-bisPSMA administered as a single cycle has a favorable safety profile. AEs were reported as related to 67Cu-SAR-bisPSMA in 8 out of the 15 trial participants (all grades). No AEs were reported as related to 64Cu-SAR-bisPSMA. Most AEs related to 67Cu-SAR-bisPSMA were grade 1 or 2. The most common grade 3 AE was anaemia, reported in 2/15 participants (13%) (Table 1).
Grade 3 n = 15 (100%) | |
Any drug-related (to 67Cu-SAR-bisPSMA) | 3 (20) |
Occurring in at least 1 participant | |
Anaemia | 2 (13) |
Thrombocytopenia | 1 (7) |
Leukopenia | 1 (7) |
Lymphopenia | 1 (7) |
Table 1. Frequency of grade 3 AEs related to 67Cu-SAR-bisPSMA observed in 3 of 15 participants. The same participant may have developed more than one AE.
Based on the safety profile observed in the first 3 cohorts of the SECuRE trial, a change to the dosing schedule of cohort 4 from "2 doses" to "up to 4 doses" has been approved by the SRC. The amendment to the protocol is currently underway, which will be submitted to the US Food and Drug Administration (FDA) and respective Institutional Review Boards (IRBs) for implementation. This will allow patients who are benefiting from 67Cu-SAR-bisPSMA to receive 2 additional doses under the SECuRE trial in cohort 4 (up to 4 doses in total).
Dosimetry
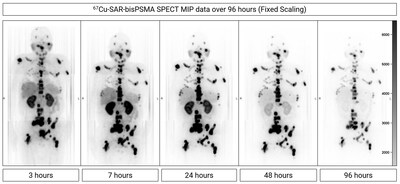
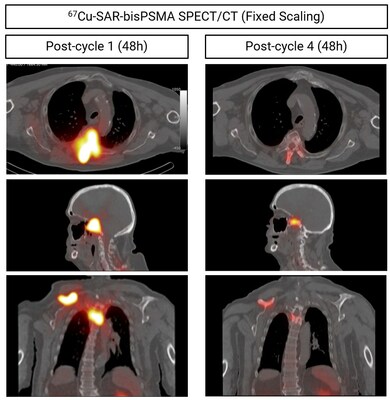
Dosimetry has been incorporated into the SECuRE trial in order to assess the absorbed dose (mGy/MBq) in organs and lesions by 67Cu-SAR-bisPSMA using single-photon emission computed tomography (SPECT) at various timepoints post 67Cu-SAR-bisPSMA injection. Dosimetry images showed prolonged tumour retention of 67Cu-SAR-bisPSMA with kidney clearance (Figure 3).
Dose escalation in the current SECuRE study protocol is based on observed organ toxicity rather than arbitrary organ absorbed dose thresholds derived from external beam radiation therapy. Nevertheless, the dosimetry results show high uptake and retention of 67Cu-SAR-bisPSMA in lesions and good clearance from organs, supporting the administration of multiple doses of 67Cu-SAR-bisPSMA at the highest dose level (12GBq).
67Cu-SAR-bisPSMA multi-dose experience: US Expanded Access Program (EAP)
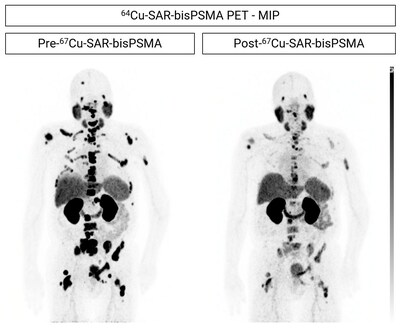
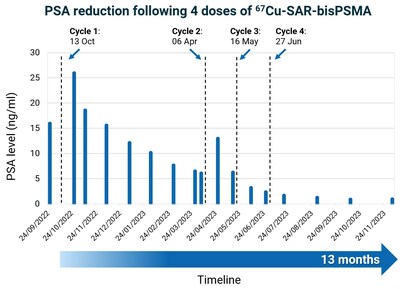
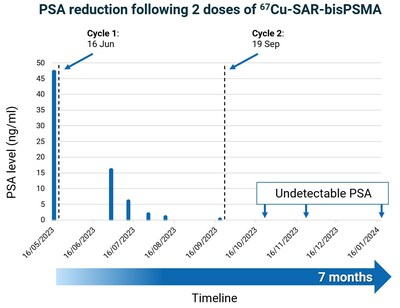
Some participants in the SECuRE trial who have shown clinical benefit during the dose escalation phase of the study received additional cycles of 67Cu-SAR-bisPSMA under the EAP, as outlined in previous announcements[2],[3]. The first participant from the SECuRE trial to receive additional doses under EAP had achieved a PSA reduction of 61.7% following the administration of a single dose of 4GBq of 67Cu-SAR-bisPSMA in cohort 1 of the trial (PSA reduction from 15.9 to 6.5 ng/ml 6 months post-injection). The treating clinician requested 3 additional doses under the EAP, and the patient continued to show further decrease in PSA levels, to 1 ng/ml (last follow-up). The overall reduction in PSA after 4 doses of 67Cu-SAR-bisPSMA was 93.7% (Graph 1). This level of reduction has been recently confirmed at the patient's last follow-up, 13 months after the first dose of 67Cu-SAR-bisPSMA. The PSA reduction has also been accompanied by a decrease in uptake of 67Cu-SAR-bisPSMA, measured by SPECT (Figure 4). No adverse events were reported related to either product, 64Cu-SAR-bisPSMA or 67Cu-SAR-bisPSMA.
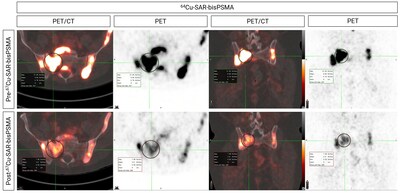
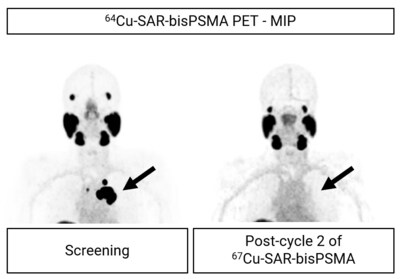
Another participant from the SECuRE trial (cohort 2, single dose of 8GBq of 67Cu-SAR-bisPSMA) showed a reduction in PSA level of 99.4%. The treating clinician requested a second dose of 67Cu-SAR-bisPSMA under EAP, which led to a further reduction of the patient's PSA to undetectable levels (Graph 2). A radiographical response was also noted: prostate cancer was undetectable using PET and a near complete response (CR) to treatment at the last assessment was observed using Response Evaluation Criteria In Solid Tumours 1.1 (RECIST) assessment. The reduction in size of one lymph node missed the CR cut-off by 2 mm (reduction in diameter post 67Cu-SAR-bisPSMA cycles from 27 to 12 mm) at time of imaging (Figure 5). The patient experienced only mild or moderate non-hematological AEs (dry mouth, altered taste and fatigue) which required no treatment and either improved or resolved over time.
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "We remain incredibly excited about our SAR-bisPSMA programs. It is remarkable to see these patients, who have failed so many lines of therapy in the past, now respond to treatment with 67Cu-SAR-bisPSMA and with such a favourable safety profile. Based on these promising results, we have just opened the multi-dosing phase at the highest dose of 12GBq and all slots for the first part of cohort 4 have now been allocated, aiming to have the first participant treated in this cohort within weeks.
"We observed PSA responses in all cohorts, including at the lowest dose level of 4GBq of 67Cu-SAR-bisPSMA. Analysis of cohorts 2 and 3 showed even greater responses, and although we are only weeks from the last participant receiving therapy, we have already seen PSA reductions of greater than 35% in all but two participants to date who had received multiple lines of therapy (including 225Ac-based therapy) and had high PSA levels prior to receiving 67Cu-SAR-bisPSMA. As observed in some patients, this fall in PSA may continue for many months after treatment. What is also exciting at this early timepoint is that almost one in two participants in cohorts 2 and 3 so far has experienced a drop in PSA levels of greater than 80% after a single dose of 67Cu-SAR-bisPSMA. Once again, although it is early after the last participant has been treated, these results compare very favorably to those in patients receiving multiple doses of other radioligand products that are commercially available or in development. Coupled with a favorable safety profile, with most adverse events being only mild or moderate and approximately half recovering to date within weeks after the last treatment, we are very excited to continue the development of 67Cu-SAR-bisPSMA.
"Given the outstanding data in the trial so far, what is most important for us now is the longevity of response, and the results we have seen from 2 case studies conducted under the EAP are extremely encouraging. Based on the clinical benefit seen in the SECuRE trial, clinicians have requested additional doses of 67Cu-SAR-bisPSMA under the EAP.2,3 These 2 patients received 1 or 3 additional doses of 67Cu-SAR-bisPSMA at 8GBq or 4GBq, respectively. They have failed multiple lines of therapy prior to being treated with 67Cu-SAR-bisPSMA and have had a dramatic response, with only few mild or moderate side effects from the treatment, also demonstrating great efficacy with the multi-dosing treatment. We look forward to replicating the case study results in the multi-dose phase of the trial at the higher dose of 12GBq. If we observe a similar response in larger patient numbers, 67Cu-SAR-bisPSMA may become the gold standard therapeutic agent for patients with mCRPC once approved."
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) Technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR Technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are unregistered products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide[4]. The American Cancer Institute estimates in 2024 there will be 299,310 new cases of prostate cancer in the US and around 35,250 deaths from the disease[5].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References |
[1] Clinicaltrials.gov Identifier: NCT04868604. clinicaltrials.gov/ct2/show/NCT04868604 |
[2] Clarity's theranostic prostate cancer trial advances to highest dose level. Announcement. 10 Aug 2023. claritypharmaceuticals.com/news/secure_cohort3/ |
[3] First patient with metastatic prostate cancer to receive 2 doses of Cu-67 SAR-bisPSMA achieves undetectable PSA level. Announcement, 30 Nov 2023. claritypharmaceuticals.com/news/sar-bispsmaundetectablepsa/ |
[4] Global Cancer Statistics 2020. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 |
[5] American Cancer Society Key Statistics for Prostate Cancer. cancer.org/cancer/prostate-cancer/about/key-statistics.html |
For more information, please contact: | |
Clarity Pharmaceuticals | |
Dr Alan Taylor | Catherine Strong |
Executive Chairperson | Investor/Media Relations |
+61 406 759 268 |
This announcement has been authorised for release by the Executive Chairperson.

SOURCE Clarity Pharmaceuticals
-
After Earnings, Is Alphabet Stock a Buy, a Sell, or Fairly Valued?
-
When Will the Fed Start Cutting Interest Rates?
-
What’s the Difference Between the CPI and PCE Indexes?
-
Powell Unfazed By Sticky Inflation, but Rate Cuts Are Far Off
-
After Earnings, Is Microsoft Stock a Buy, a Sell, or Fairly Valued?
-
Best- and Worst-Performing Stocks of April 2024
-
Magnificent 7 Stocks Earnings Updates: AI Remains the Focus
-
Small-Cap and Value Stocks Are Undervalued
-
4 Utility Stocks to Play the AI Data Center Boom
-
Albemarle Earnings: We Expect Improved Results In the Rest of Year Following Cyclically Low Profits
-
Novo Nordisk Earnings: Raised Fair Value Estimate Still a Contrast to Market Overenthusiasm
-
After Earnings, Is Verizon Stock a Buy, a Sell, or Fairly Valued?
-
Look Inside Berkshire Hathaway’s Portfolio Before Its Annual Meeting
-
How to Invest Like Warren Buffett
-
Cognizant Earnings: Improved Profitability Buttresses Results as Customer Spending Remains Muted
-
10 Top-Performing Dividend Stocks of the Month